Spinal Anaesthesia in children: Comparative study of isobaric levobupivacaine 0.5% with or without clonidine
Abstract
Background & Objectives: Spinal anaesthesia reduces the incidence of morbidity that follows general anaesthesia in children with good efficacy and safety record. Racemic bupivacaine is the widely used local anaesthetic for paediatric spinal anaesthesia. Local anaesthetic levobupivacaine has an equivalent potency to that of racemic bupivacaine but with a greater safety margin. Limited duration of surgical anaesthesia is however, one of the major deterrents for widespread use of spinal anesthesia in children. Clonidine lengthens spinal anaesthesia in adults and in children. We conducted a prospective, randomized clinical trial to study the effect of the addition of clonidine to 0.5% isobaric levobupivacaine induced spinal anaesthesia in children undergoing infra-umbilical procedures.
Methods: Sixty patients between 5 to 10 years, posted for infra-umbilical surgeries were given spinal anaesthesia with either 0.5% plain isobaric levobupivacaine (5-15 kg – 0.4 mg/kg, 15-40 kg – 0.3 mg/kg) or levobupivacaine (5-15 kg – 0.4 mg/kg, 15-40 kg – 0.3 mg/kg) with clonidine (1μg/kg). Blockade characteristics and duration of analgesia were recorded along with mean arterial pressure, heart rate, respiratory rate and SpO2.
Results: Satisfactory surgical anaesthesia was achieved in all 60 patients with comparable mean arterial pressure, heart rate, respiratory rate and SpO2. Block extension was 2 segments higher in patients given intrathecal clonidine. Duration of spinal anaesthesia (2 segment regression) increased from (90.3 ± 7.2 min) in the control group to (143.5 ± 8.7 min) in the group receiving clonidine 1μg/kg (p<0.001) and postoperative analgesia from 228 ± 14 min to 360 ± 31min (p<0.001). Sedation was the most common side effect observed in children receiving intrathecal clonidine.
Conclusion: We conclude that clonidine 1μg/kg provides a significant improvement in spinal anesthesia and analgesic duration without undesirable side-effects.
Key words: Spinal anaesthesia, children, levobupivacaine, clonidine
Introduction
Spinal anaesthesia has been found as a good alternative to general anaesthesia, producing an intense and uniformly distributed sensory block with good muscle relaxation(1) and thus becoming increasingly popular as anaesthetic technique of choice for children undergoing infraumbilical surgical procedures(2). It is as simple, safe and inexpensive in children as in adults.
Bupivacaine is the most common local anaesthetic for spinal anaesthesia in children(2,3). Levobupivacaine, the S(-) enantiomer of bupivacaine, has less potential for cardiovascular and central nervous system toxicity than bupivacaine(4,5). It has a clinical efficacy equivalent to bupivacaine(6,7,8) and been used for spinal anaesthesia both in adults(6,7,8) and in children(9).
As an adjunct to spinal anaesthesia, clonidine is known to prolong both sensory and motor blockade in adults(10,11) and in infants(12) induced by bupivacaine, with reports of using clonidine for prolongation of levobupivacaine spinal anaesthesia in adults.
We conducted a prospective clinical trial in children between 5 to 10 years, undergoing infraumbilical surgeries to investigate the duration of surgical blockade and postoperative analgesia provided by clonidine added to levobupivacaine spinal anaesthesia. Haemodynamic response, respiratory and sedative effects of spinal clonidine was studied as the secondary aim.
Methods
Sixty healthy children aged 5 to 10 years, scheduled for lower abdominal, urological and lower limb surgeries under spinal anaesthesia were enrolled. All the children were included unless they had any contraindications for spinal anaesthesia, respiratory tract infection, allergy to levobupivacaine or clonidine. The study was approved by our institutional ethics committee and parental informed consent was obtained after detailed explanation of the procedure.
The patients were admitted a day prior to the operation and fasted for 5 hours before anaesthesia. One hour before anesthesia, all the children were given oral Midazolam 0.5 mg/kg as sedative premedication and Prilox® (Lidocaine 2.5% + Prilocaine 2.5%) ointment was applied at the lumbar puncture site and site for achieving the intravenous access. All the children were the monitored by an anaesthesiologist or an anaesthetic assistant.
Once in the operation room, intravenous infusion with Ringers Lactate was started, Oxygen given through facemask and routine monitoring as SpO2, NIBP and ECG was applied (Philips MP 50). A bolus dose of Inj. Propofol 0.5-1mg/kg was administered intravenously to maintain immobilization during lumbar puncture. Lumbar puncture was performed in the lateral decubitus position at the L4-5 interspace with a 4 cm, 25G spinal needle using midline approach. Correct placement was confirmed by a free aspiration of CSF and the anaesthetic solution was injected over 3-4 s (~0.25 ml/s) for spinal anaesthesia. The patients were randomly allocated to 1 of the two groups according to the study drug. Group A received plain 0.5% isobaric levobupivacaine (5-15 kg – 0.4 mg/kg, 15-40 kg – 0.3 mg/kg) and Group B received plain 0.5% isobaric levobupivacaine (5-15 kg – 0.4 mg/kg, 15-40 kg – 0.3 mg/kg) with clonidine (1 μg/kg) intrathecally. After injection the patients were immediately made supine and remained at the same position for the rest of the surgery. Oxygen supplementation 3 l/min was administered through facemask applied.
The onset and the extent of the sensory block were assessed by observing either facial expression or movement of the child to bilateral non traumatic pin prick every 2 min after completion of intrathecal injection for first 10 min and every 5 min thereafter. The onset was considered as the complete loss of sensation at L1. Motor blockade was assessed according to the Bromage scale (0- Free movement of leg & feet, 1 – knee flexion less, 2 – unable to flex knees, ankle & feet flexion present, 3 – unable to flex knee, ankle or move toes). An appearance of Grade 1 motor block was taken as the onset of motor blockade and Grade 3 as complete motor block. Surgery was allowed to commence on attaining a sensory level of T10 or above. The routine monitoring of mean arterial pressure, heart rate, respiratory rate and SpO2 was recorded for the same intervals and every 10 min during the surgery or till 90 min and then every half-hourly henceforth and in the post-anaesthetic care unit for each patient.
Hypotension, defined as decrease in systolic >30% from baseline, was treated with an infusion of Ringers lactate solution 10 ml/kg and with IV ephedrine 3 mg. Bradycardia, defined as a heart rate less than lower limit of normal for age (13) and a rate < 70 beats/ min was treated with atropine 0.01 mg/kg. Ringers lactate was administered intravenous as maintenance and replacement fluid, by the ‘4-2-1 rule’. Arrangement of blood transfusion was done in case of need.
After surgery all patients received paracetamol rectally, 300 mg paracetamol given rectally to children < 10 kg, 500 mg between 10 – 15 kg and 650 mg to children > 15 kg. They were then transferred to the post anaesthetic care unit for continuous monitoring of vital signs and regression of block till full recovery and then transferred to respective surgical units and followed up for 24 hr. The time taken for the dermatomal level to regress from the highest level by two segments from spinal injection was noted as time of 2-segment sensory regression and the time to regress to T12 from the highest dermatomal level was noted as time to regression to T12. The total duration of motor block was the time taken from injection of drug to regression of motor block to Grade 0 Bromage Scale. Sedation was recorded upon entering the operation room and along with vital signs throughout. Ramsay’s Sedation Score (1 – Awake calm, 2 – Asleep arousable by verbal contact, 3 – Asleep arousable by physical contact, 4 – Asleep, not arousable) was utilized for recording degree of sedation. In addition, SpO2, respiratory rate and evidence of respiratory distress were carefully evaluated.
Post operative pain was assessed using FLACC score. The time to the first rescue analgesic were recorded as when FLACC score remained > 4 for two consecutive intervals of 10 minutes. The duration of complete analgesia was taken as the time interval from the time of intrathecal injection till Scores remained 0 and the duration of analgesia was taken till the reception of rescue analgesic.
The sample size was determined after undergoing a pilot study and the number of patients to be included was calculated from the mean and standard deviation on the assumption of a minimum difference of increase of 50% in the duration of sensory block between the two groups. 22 patients were required in each group in order to have a 90% chance at the two-tailed 0.05 level of significance to detect a difference between the groups and we included 30 patients in each group in our study.
Data obtained were analyzed with the Statistical software STATA version 13.1. Statistical tests for categorical data were χ2 test. For continuous data, analysis of variance (ANOVA) and the Student’s t-test were applied. The level of significance was set at P<0.05.
Results
There was no significant difference in the patient demographics. Distribution of patients according to type of surgeries was also comparable (Table 1).
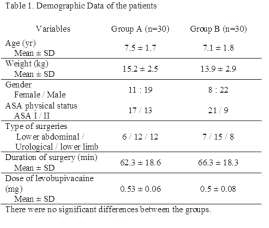
Sensory block appeared within 5 min in all patients except for 10 in Group A, with a time for maximum cephalic spread being significantly more in Group A than in Group B. The cephalic spread of the sensory block was a median two segments higher in Group B with a significantly longer duration of sensory regression by two segments and regression to T12 (Table 2).
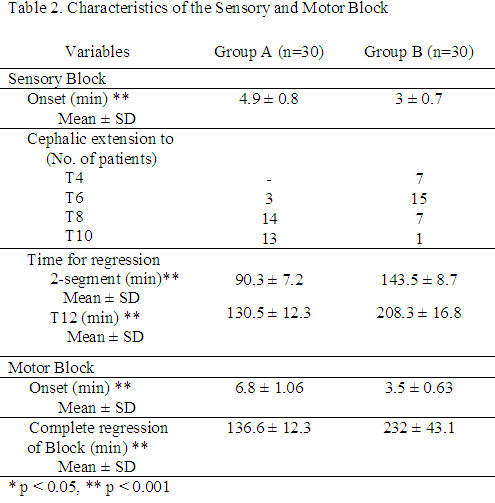
There was no difference between the two groups in motor block, however, the time to achieve the blockade was earlier and time to recovery of motor block was significantly longer in Group B compared with that in Group A. Hence, clonidine not only enhanced onset but also provided an extended duration of surgical anaesthesia. There was no difference in the need for rescue analgesics between the two groups. However, the time to the first dose of rescue analgesic was longer in Group B than in Group A. (Table 3)

Mean arterial pressure and heart rate remained lower than preoperative levels in Group B compared to Group A (Figure 1 & 2). Two patients in Group B had a fall in pulse rate to <70/min, observed at 35 min and 50 min. No significant changes in respiratory rate and SpO2 were noted in any children of either of the groups at any time.
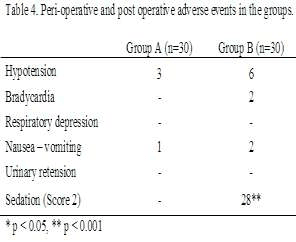
There was no difference between the two groups regarding the incidence of perioperative adverse events except for a deep sedation (Ramsay sedation Score 2), exhibited by almost 28 (93%) children in Group B in this respect. This was observed beyond 30 minutes of instillation of the drug spinally and resolved with the regression of the block (Table 4).

Discussion
Spinal anaesthesia in children is gaining popularity due of innumerable advantages like adequate anaesthesia without polypharmacy, endotracheal intubation and respiratory support, minimal biochemical and physiological disturbances, adequate postoperative analgesia, absence of postoperative nausea & vomiting with rapid return of feeding. However, the shorter duration of action of spinal anaesthetics is a major limiting factor (14, 15). We observed the effect of the addition of clonidine 1mcg/kg to intrathecal 0.5% isobaric levobupivacaine in children between 5 – 10 years decreased time to readiness to surgery, prolonged duration of sensory block i.e. 2 segment regression and motor block by 60% and postoperative analgesia by 50%. Addition of clonidine could make levobupivacaine a feasible option in cases where the procedure is expected to last for more than what can be covered by a single intrathecal dose of levobupivacaine.
Results confirm with the findings by Kaabachi O et al, using clonidine 1mcg/kg as an adjuvant to intrathecal isobaric bupivacaine for spinal anaesthesia in adolescents (16). The potential for toxicity with levobupivacaine is less than with racemic bupivacaine(17) and considered to be a safe alternative to bupivacaine especially in peripheral blocks were large doses of anaesthetics are required. In spinal anaesthesia, wherein a small dose produces effective anaesthesia with a less potential for toxicity, the safety of levobupivacaine over bupivacaine can be overlooked. However, deposition of drug into the close proximity to the spinal nerves and cord is worth taking into consideration of using a drug with less potential for toxicity (9). In a study to determine the clinical efficacy and MLAD (minimum local anaesthetic dose) of levobupivacaine for spinal anesthesia in lower abdominal surgery suggested an equivalent potency to racemic bupivacaine (3).
Readiness to surgery was quicker as observed with the earlier onset and spread of sensory block in children receiving clonidine intrathecally along with levobupivacaine in the present study. Motor block was complete in all the patients of the study. However, the cephalic spread of sensory block in about one-fourth of the children receiving clonidine was above T6. Rochette A et al (18) and Kaabachi O et al (16) did not find any difference in the extent of the sensory block with the addition of various amounts of clonidine to isobaric bupivaciane. This difference could be attributed to the method of pin-prick, which is a subjective method used to test the spread of anaesthesia in sedated patients in the present study. The transcutaneous electrical stimulation, an objective method unavailable with us, have been used in studies for accurate testing, and may be better than pin-prick.(9, 18)
Clonidine 1mcg/kg is an appropriate dose as an adjuvant to spinal dose of levobupivacaine as observed in this study with a 60% increase in duration of surgical anaesthesia and 50% increase in post operative analgesia. Reports of varying doses of clonidine with bupivacaine have suggested 1mcg/kg increased duration of block two-fold compared to plain isobaric bupivacaine not associated with haemodynamic or clinically respiratory alterations. Increasing dosage to 2 mcg/kg increased incidence of side effects with a similar duration of block (18, 19). It was observed that mean arterial pressure and heart rate remained below baseline values, not warranting aggressive intervention when clonidine was added to levobupivacaine, returning to preoperative value with the regression of the block. However, hypotension was more frequent with 20%, in the patients receiving clonidine and 10% in the control group. 7% depicted bradycardia in the clonidine group. Rochette et al too, reported that number of patients who experienced mean arterial pressure < 40 mmHg were more in those who received intrathecal clonidine 1mcg/kg (18). Kaabachi et al (16) too, noted that, incidence of adverse events with spinal clonidine as an adjuvant was high but, without causing severe adverse effects. The sedative effect of clonidine is well known. High sedation scores were significantly more often observed in children receiving clonidine which resolved with the block (18) with no respiratory impairment in the peri-operative period. A calm and sedated child with no significant clinically haemodynamic and respiratory alteration could be an advantage in procedures done under spinal anaesthesia.
In conclusion, the present study indicates that adding clonidine 1μg/kg to isobaric levobupivacaine 0.5% hastens onset of spinal block, doubles spinal anaesthesia and postoperative analgesia in children between 5 – 10 years, without imparting unwanted haemodynamic, respiratory and adverse effects.
References:
- Wolf AR, Doyle E, Thomas E. Modifying infant stress responses to surgery to major surgery: spinal vs extradural vs opioid analgesia. Paediatr Anaesth 1998;8:305-11.
- Kokki
H. Spinal anaesthesia in infants and children. Baillieres Clin Anaesthesiol
2000;14:687-707.
Puncuh F, Lampugnani E, Kokki H. Spinal Anaesthesia in Paediatric patients. Current Opinion in Anaesthesiology 2005, 18:299-305 - Frawley G, Smith KR, Ingelmo P. Relative potencies of bupivacaine, levobupivacaine and ropivacaine for neonatal spinal anaesthesia. Br J Anaesth 2009;103:731-8.
- Aberg G. Toxicological and local anaesthestic effects of optically active isomers of two local anaesthetic compounds. Acta Pharmacol Toxicol 1972;31:273-86.
- Bardsley H, Gristwood R, Baker H, Watson N, Nimmo W. A comparison of the cardiovascular effects of levobupivacaine and rac-bupivacaine following intravenous administration to healthy volunteers. Br J Clin Pharmacol. Sep 1998;46(3):245–9.
- Burke D, Kennedy S, Bannister J. Spinal anaesthesia with 0.5% S(-)-bupivacaine for elective lower limb surgery. Reg Anaesth Pain Med 1999;24:519-23.
- Glaser C, Marhofer P, Zimpfer G, et al. Levobupivacaine versus racemic bupivacaine for spinal anesthesia. Anesth Analg 2002;94:194-8.
- Alley EA, Kopacz DJ, McDonald SB, Liu SS. Hyperbaric spinal levobupivacaine: a comparison to racemic bupivacaine in volunteers. Anesth Analg 2002;94:188-93.
- Kokki H, Ylönen P, Heikkinen M, Reinikainen M. Levobupivacaine for pediatric spinal anesthesia. Anesth Analg 2004;98:64-7.
- Nlemi L. Effects of intrathecal clonidine on duration of bupivacaine spinal anaesthesia, haemodynamics, and postoperative analgesia in patients undergoing knee arthroscopy. Acta Anaesthesiologica Scandinavica October 1994;38(7):724–8.
- Jamliya RH, Vansola R, Shah BJ, Chauhan L. Effect of clonidine addition to hyperbaric 0.5% bupivacaine for spinal anaesthesia in lower limb surgery [A comparative study]. NJIRM 2012;3(1):113-119
- Cao JP, Miao XY, Liu J, Shi XY. An evaluation of intrathecal bupivacaine combined with intrathecal or intravenous clonidine in children undergoing orthopedic surgery: a randomized double-blinded study. Paediatr Anaesth 2011;21(4):399-405
- Hartman M E, Chieftez I M. Nelson Textbook of Anaesthesia. Pediatric Emergencies and Resuscitation 279-96. 19th edition, Philadelphia PA, Elsevier Saunders, 2011, Print
- Puncuh F, Lampugnani E, Kokki H. Spinal anaesthesia in paediatric patients. Curr Opin Anaesthesiol 2005;18:299-305.
- Gupta A, Saha U. Spinal anesthesia in children: A review. Journal of Anaesthesiology Clinical Pharmacology 2014;30(1):10-18
- Kaabachi O, Zarghouni A, Ouezini R, Abdelaziz AB et al. Clonidine 1mcg/kg is a safe and effective adjuvant to plain bupivacaine in spinal anaesthesia in adolscents. Anesth & Analg 2007;105(2):516-19
- Foster RH, Markham A. Levobupivacaine: A review of its pharmacology and use as a local anaesthetic. Drugs 2000:59:551-9
- Rochette A, Raux O, Troncin R, Dadure C et al. Clonidine prolongs spinal anesthesia in newborns: A prospective dose-ranging study. Anesth Analg 2004;98:56-9
- Kaabachi O, Ben Rejeb A, Mebazza M, Safi H et al. Spinal anaesthesia in children: comparative study of hyperbaric bupivacaine with or without clonidine. Ann Fr Anesth Reanim 2002;21:617-21